Ozone: What It Is & How It Affects You - Learn Now!
Is there a silent protector, an invisible shield, constantly working to safeguard life on Earth? The answer, strikingly, is yes: the ozone layer, a critical component of our planet's atmosphere, is essential for survival.
Ozone, or trioxygen, is a molecule comprising three oxygen atoms. This seemingly simple structure belies its profound importance. In the upper atmosphere, the stratosphere, ozone acts as a filter, absorbing a significant portion of the sun's ultraviolet (UV) radiation. This protective function is paramount, shielding living organisms from the damaging effects of UV rays, which can cause skin cancer, cataracts, and other health problems, as well as damage plant life and ecosystems. The ability of ozone to absorb UV radiation is a result of its molecular structure, which allows it to interact with and break down the high-energy photons in UV light.
However, the same molecule that acts as a celestial guardian can also be a terrestrial threat. While beneficial in the stratosphere, ozone becomes a harmful pollutant near the Earth's surface. At ground level, ozone is a primary component of smog and can cause respiratory problems, irritate the eyes and throat, and worsen existing conditions like asthma. This duality highlights the complex nature of ozone and its varying effects depending on its location within the atmosphere.
- How To Know Signs A Man In Love With You Simplified
- Essential Guide To The White Tip With Gn Insights And Features
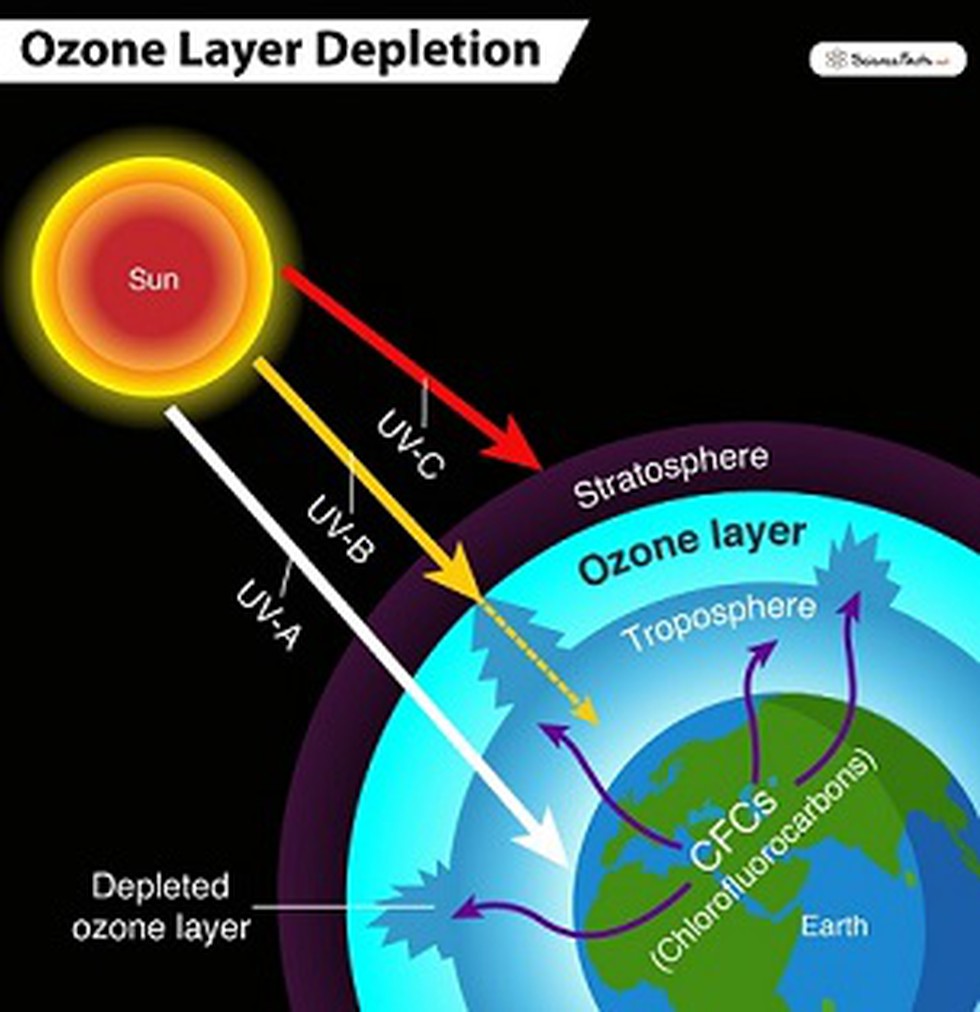
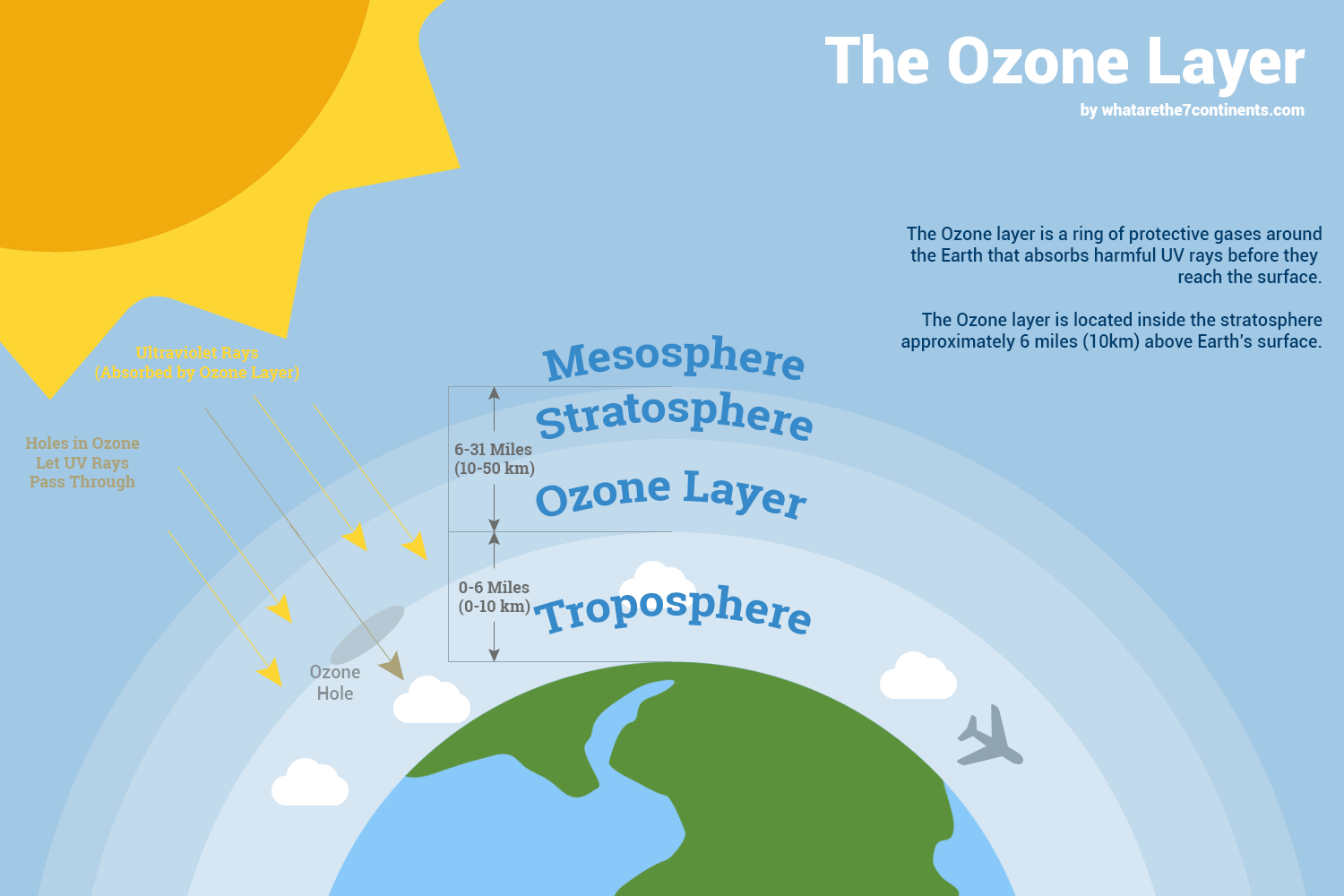
The ozone layer, a region of the stratosphere located between roughly 15 and 35 kilometers (9 and 22 miles) above the Earth's surface, is not a solid, distinct layer. Instead, its a region where ozone molecules are relatively concentrated compared to other parts of the atmosphere. This concentration, though still sparse about three ozone molecules for every 10 million air molecules is sufficient to absorb a significant amount of UV radiation.
The significance of the ozone layer lies in its role in protecting life on Earth from the harmful effects of ultraviolet radiation. This radiation can damage and disrupt DNA, leading to mutations and various health problems. The ozone layer acts as a natural sunscreen, absorbing most of the sun's harmful UV-B and all of the UV-C radiation, allowing life to flourish on Earth. Without this protection, the planet would be a far more hostile environment.
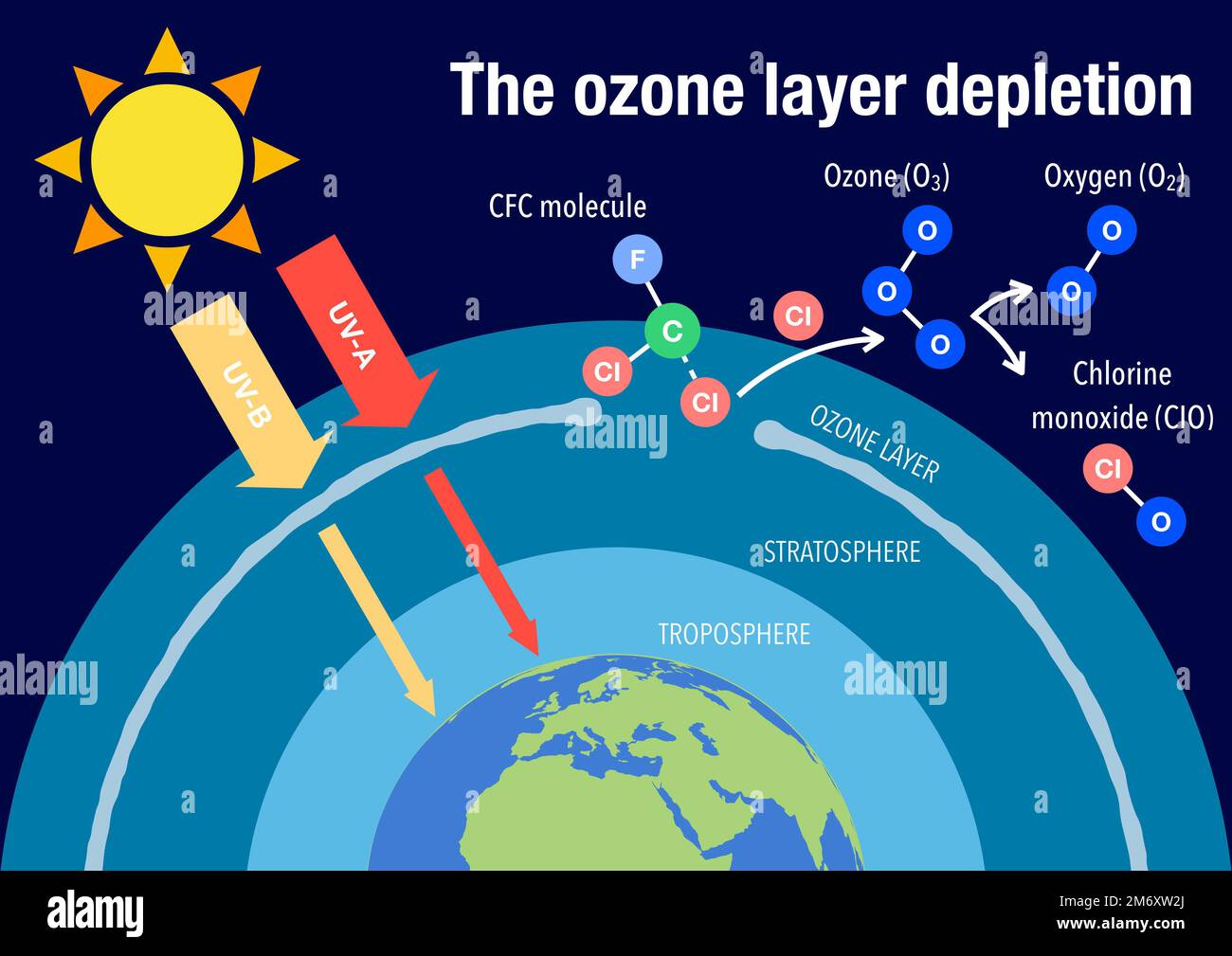
The formation of ozone is a dynamic process, primarily driven by the interaction of UV radiation with oxygen molecules (O2). This process, known as photolysis, breaks down oxygen molecules into individual oxygen atoms. These free oxygen atoms then combine with other oxygen molecules to form ozone (O3). The reverse process also occurs, with ozone molecules absorbing UV radiation and breaking down back into oxygen molecules and oxygen atoms. This constant cycle maintains the ozone layer and its protective function.
- Fortnite Hime Skin A Complete Guide To Mastering The Aesthetic
- The Ultimate Guide To Cool Blonde Hair Color Styles Tips And More
Human activities have significantly impacted the ozone layer, leading to its depletion. The release of chemical compounds containing chlorine and bromine, such as chlorofluorocarbons (CFCs) and halons, used in refrigerants, aerosols, and fire extinguishers, has been a major contributor to ozone depletion. These chemicals, once released into the atmosphere, migrate to the stratosphere, where they are broken down by UV radiation, releasing chlorine and bromine atoms. These atoms act as catalysts, triggering a chain reaction that destroys ozone molecules. This depletion is most pronounced in the polar regions, particularly over Antarctica, where the "ozone hole" has become a well-documented phenomenon.
The consequences of ozone depletion are far-reaching. Increased UV radiation reaching the Earth's surface can lead to increased rates of skin cancer, cataracts, and immune system suppression in humans. It can also damage plant life, disrupting ecosystems and affecting food production. Marine ecosystems are also vulnerable, with increased UV radiation potentially harming phytoplankton, which are the base of the marine food chain. The environmental and health risks associated with ozone depletion prompted international action to address the problem.
The Vienna Convention for the Protection of the Ozone Layer, established in 1985, provided a framework for international cooperation on ozone depletion. This was followed by the Montreal Protocol in 1987, a landmark agreement that aimed to phase out the production and consumption of ozone-depleting substances. The Montreal Protocol has been remarkably successful, with the phasing out of CFCs and other harmful chemicals leading to a gradual recovery of the ozone layer. Scientists have observed signs of recovery, with projections indicating that the ozone layer will be restored to pre-1980 levels in the coming decades. This recovery is a testament to the power of international cooperation and the effectiveness of environmental regulations.
The monitoring of the ozone layer and the assessment of the ongoing impacts of ozone depletion and the effectiveness of international treaties is done by scientific studies that are regularly carried out and have been the subject of reports, such as the one released in early 2023, which is a testament to the impact of international collaboration and environmental restrictions.
While the ozone layer is recovering, vigilance and continued adherence to the Montreal Protocol are essential. The long atmospheric lifetimes of some ozone-depleting substances mean that it will take time for the ozone layer to fully recover. Furthermore, the potential for new threats to the ozone layer, such as the release of new chemicals or changes in climate patterns, necessitates ongoing monitoring and research. Ozone depletion also highlights the broader issue of human impacts on the environment and the need for sustainable practices to protect the planet.
The story of ozone is a powerful reminder of the interconnectedness of life on Earth and the importance of environmental stewardship. Ozone, a molecule that exists in both the stratosphere and the troposphere, acts as a protector, but, can become a pollutant depending on its location in our atmosphere. The success of the Montreal Protocol serves as a model for international cooperation in addressing other environmental challenges. The future of the ozone layer, and the health of the planet, depends on our continued commitment to protecting this vital shield.
The formation of ozone is connected with emissions of volatile organic compounds (VOCs) and nitrogen oxides (NOx). These are regulated by air quality standards to keep ozone levels within acceptable limits. The presence of ozone can impact the air quality for human and environmental health. It is essential to understand and to protect ourselves by taking appropriate precautions such as avoiding strenuous outdoor activities when ozone levels are high.
Article Recommendations
- Choosing Between Primer Or Sunscreen First The Ultimate Guide
- Top Picks For Gentle Shampoo For Color Treated Hair Enhance Amp Protect
Detail Author:
- Name : Eugene Effertz
- Username : marianne.yundt
- Email : joyce66@lemke.org
- Birthdate : 1973-04-03
- Address : 79691 Nolan Ports Suite 668 Sierrachester, NE 52978-6606
- Phone : 910.202.8617
- Company : Hayes, Koelpin and Mohr
- Job : Vice President Of Human Resources
- Bio : Consequatur eligendi dolores qui molestias. Rerum voluptatibus iusto ut id ea. Ratione quidem sequi similique nihil est ea quod voluptates.
Socials
linkedin:
- url : https://linkedin.com/in/lnolan
- username : lnolan
- bio : Necessitatibus consequatur cum et et occaecati.
- followers : 2253
- following : 1579
twitter:
- url : https://twitter.com/lula_real
- username : lula_real
- bio : Sed expedita maiores quia quia praesentium. Consequatur perferendis facere veritatis a rerum. Sit velit ullam illum dolorem distinctio quo voluptatem.
- followers : 5359
- following : 712
instagram:
- url : https://instagram.com/lula.nolan
- username : lula.nolan
- bio : Sunt suscipit quia veniam. Et et voluptatem vel nam est repellat.
- followers : 1275
- following : 2101
facebook:
- url : https://facebook.com/nolanl
- username : nolanl
- bio : Voluptas quia ut delectus beatae dolores mollitia. Sit quaerat culpa libero.
- followers : 4092
- following : 2436
tiktok:
- url : https://tiktok.com/@lula.nolan
- username : lula.nolan
- bio : Aut esse deserunt qui magni consequatur animi illum.
- followers : 273
- following : 1840